Sales of an important painkiller suspended. Important GIF message
The Chief Pharmaceutical Inspector (GIF) suspended the trade in paracetamol. The product turned out to be defective. Find out the details of the decision.
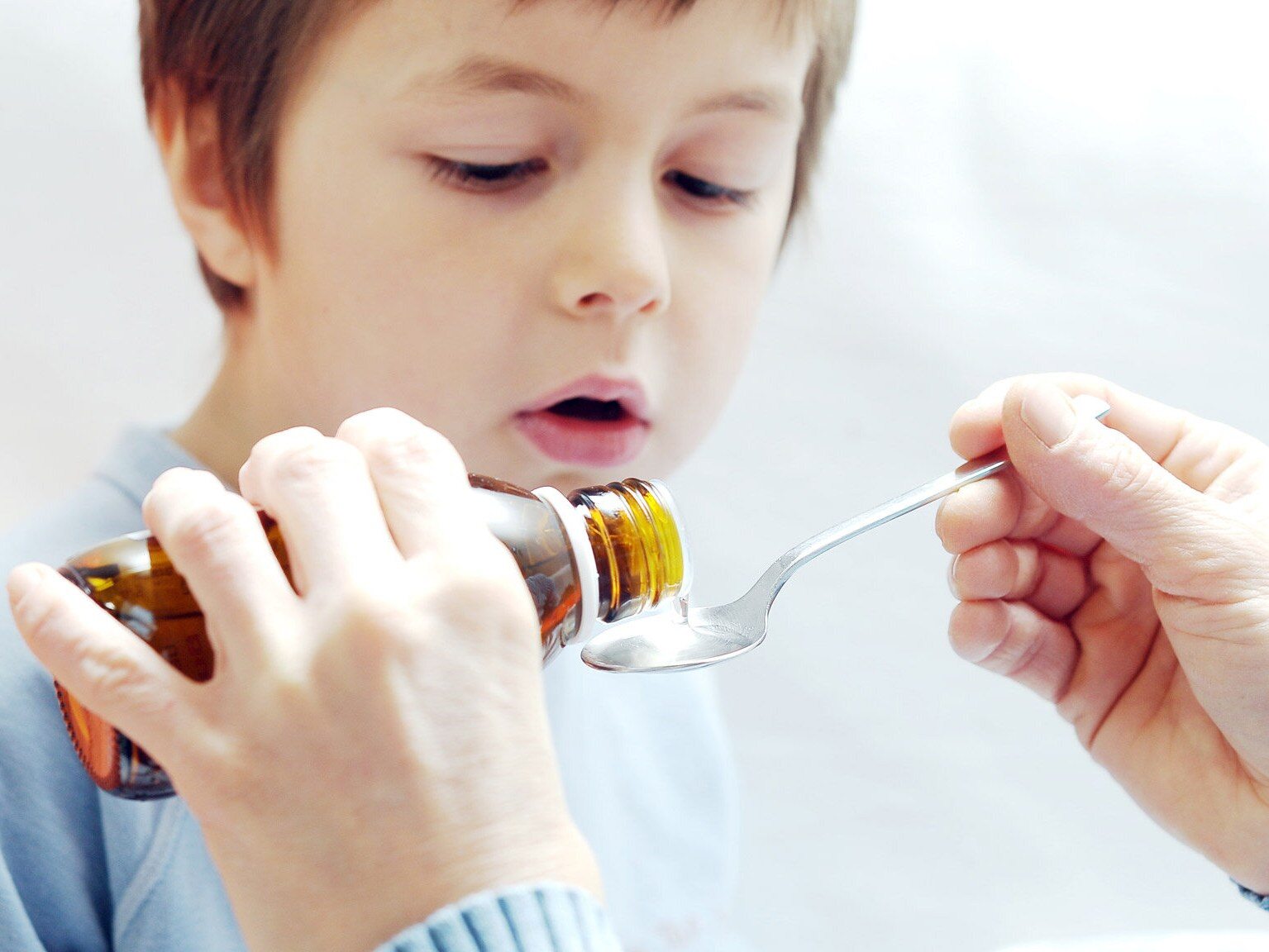
The announcement published by the Chief Pharmaceutical Inspectorate concerns a drug called Paracetamol Aflofarm (Paracetamolum). The preparation is a suspension taken orally. The main indication for its use is fever occurring in the course of the common cold. The agent is also used to treat pain of various origins of low and moderate intensity. These include headaches, toothaches, muscle and joint pain. Importantly, the drug is intended for use in children.
Aflofarm paracetamol – GIF decision
In justification of its decision, the Chief Pharmaceutical Inspectorate provided details about the medicinal product whose sale was suspended:
-
drug name: Paracetamol Aflofarm (Paracetamolum)
-
drug form and dose: oral suspension, 120 mg/5 ml, 1 bottle of 100 ml
-
GTIN: 05909991076115
-
batch number: 04AF0622, expiry date: 30/06/2025
-
batch number: 01AF1122, expiry date: 30/11/2025
-
batch number: 01AF1022, expiry date: 31/10/2025
-
batch number: 02AF1022, expiry date: 31/10/2025
-
batch number: 01AF0222, expiry date: 28/02/2025
-
responsible entity: Aflofarm Farmacja Polska Sp. z o. o. based in Pabianice
The GIF made the decision immediately enforceable. The decision of the Chief Pharmaceutical Inspector is related to the disturbing reports received by him. They were submitted, among others, by generally accessible pharmacies from the Kuyavian-Pomeranian and Lesser Poland voivodeships. They informed the pharmaceutical inspection authorities about the detection of a potential quality defect in the batches of the medicinal product indicated above.
Why will GIF suspend the sale of Paracetamol Aflofarm?
“The ground for suspending trading in the above-mentioned batches of a medicinal product, there is (…) a justified suspicion that they do not meet the quality requirements set for it, due to the impossibility of obtaining a homogeneous suspension and sediment remaining at the bottom of the bottle, despite repeated shaking of its contents. Due to the above considerations, in view of the Chief Pharmaceutical Inspector's justified suspicion that the batches of the medicinal product indicated in the decision (…) do not meet the quality requirements set for them, it has become necessary and justified to suspend their marketing,” we read in the GIF press release.